|
'World's Foremost Scientist'

Langmuir receiving the Nobel Prize in 1932.
If the SAS slogan 'Who dares wins' applies to scientists then we would expect some fireworks from a budding chemist. If not literally fireworks, then some strong stinks at least. For example, Irving Langmuir, who was to receive a Nobel Prize for chemistry, did not do things by halves. At the tender age of six, he nearly killed himself by sniffing chlorine.
It happened when Arthur, Irving's older brother by nine years, had access to a well-appointed chemistry laboratory. Probably not realising how dangerous chlorine is, he took to sometimes carrying a small bottle of the gas around with him and sniffing it. Taking the bottle home he offered Irving a smell. Irving, 'never a cautious type,' took a gulp and 'nearly strangled on the spot'. Several anxious choking days passed before! he could.breathe properly again.
Father banned further chemistry experiments, except in the classroom. Undeterred, Arthur grew up to become a successful industrial chemist and Irving gained an international reputation for both chemistry and physics.
Irving Langmuir was one of the first immensely successful industrial scientists. He was fortunate to spend most of his working life at the General Electric (GE) Research Laboratories at Schenectady in New York, where industrial research was expected not only to yield practical results but to expand knowledge as well. His early successes in improving incandescent lamps must have paid his salary for the rest of his career, and he was left free to let his imagination run riot - to the enormous benefit of electronics and science in general.
Langmuirs career path was set by his choice of postgraduate study. After graduating from Columbia University in 1903 with a degree in metallurgical engineering he left the USA for Germany where he enrolled for a PhD at the University of Gottingen. There he studied physical chemistry under Prof Walther Nernst, inventor of the Nernst lamp, who suggested that Langmuir should examine the dissociation of water vapour and carbon dioxide around glowing platinum wires. These studies not only gave Langmuir his PhD in 1906 but the basic techniques which were to flower later at the GE laboratories.
On returning to the USA he lectured at the Stevens Institute of Technology in Hoboken, New Jersey. In the summer of 1909 he took vacation employment at the GE Research Laboratories. When due to leave he was asked to stay - higher education thus lost a teacher but industrial research gained a genius.
His first task was to study thermal conduction and convection in gases at high temperatures. For this the new tungsten filaments, developed by W D Coolidge from an Austrian invention, were used. At first Langmuir felt embarrassed at the amount of time he enjoyed 'playing' but discovering nothing, but the director of the laboratories, W R Whitney, reassured and encouraged him.
Then Langmuir did discover something - namely that hydrogen gas behaves in a very peculiar way at extreme temperatures. In 1912 he explained this as the dissociation of hydrogen molecules into single atoms. It was an important scientific discovery which he used much later in his invention of the hydrogen torch, for which a patent was awarded in 1934.
One of the problems with the early tungsten filament light bulbs was that the inside of the glass blackened with time. It was widely held that a better vacuum would solve this and other problems. Langmuir could not think of any way to get an empty void emptier so he turned the problem inside out by adding extra gas so he could, once again, study the behaviour of gases.
His approach has been likened to treating a case of poisoning by giving more poison. To Langmuir it made sense because he could extrapolate back from his results to predict what would happen if a perfect vacuum was obtained. What he showed was that even the minutest amount of water vapour must be avoided. Beyond that, however, a better vacuum did not lessen the blackening, which was caused by the evaporation of tungsten. Instead of further improvements to the vacuum he suggested the opposite - filling the bulb with gas. 'You're dreaming!' Whitney told him, but allowed that he should try it. The result was today's argon-filled light bulb, a money spinner if ever there was.
His work on light bulbs inevitably led him to its closest cousin, the thermionic valve. The tungsten filament of the lamp became the early cathode of the valve and every physicist wanted to improve the emission of electrons from its surface. At the same time as Langmuir was improving the incandescent lamp, others were inventing new applications for radio valves.
When he studied the electron emission from tungsten filaments he found that, at temperatures close to the melting point of tungsten, the emission was orders of magnitude less than predicted by theory. Tracking down the cause of this mystery led him to the discovery of electron space charge, the results of which he described in a law. This recognition and theoretical understanding of space charge was one of the turning points in the development of the scientific design of thermionic devices.
Further studies led to the discovery that the addition of a tiny amount of thoria to the tungsten filament gave an enormous increase in the electron emission. Originally thorium oxide had been added in an attempt to prevent crystal growth of the tungsten metal, but Langmuir's detective work showed that a layer of thoria formed on the tungsten surface, resulting in the vast increase in electron emission. Thoriated tungsten filaments became standard, and the G E Labs at Schenectady further confirmed their international importance to what would become known as the electronics industry.
Recognition of his work came in 1920 when he was awarded the Rumford Medal of the American Association for the Advancement of Science. It was not the first, and certainly was not the last, honour he was to receive. On a visit to Japan in the mid 1930s he was described as the worlds foremost scientist.
Another way in which Langmuir influenced the design of electronic valves was through his improvements to vacuum pumps. In 1915 he examined Gaedes diffusion pump and, as was his style, gained both theoretical understanding and practical improvements. After the First World War Langmuir's mercury diffusion pump became a familiar sight in laboratories.
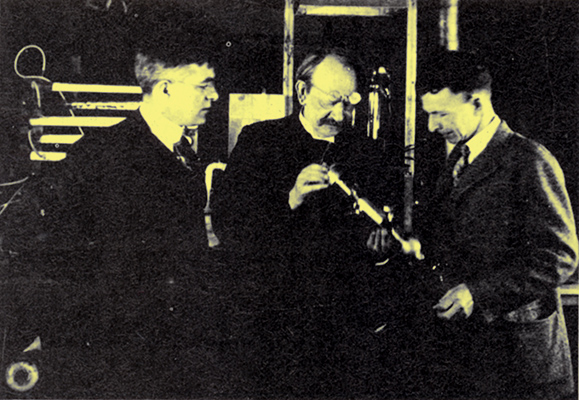
Langmuir (left) and W D Coolidge showing the pliotron to Sir J J Thomson when he visited the labs in 1923.
His work covered an incredible range of interests and he had an international reputation in subjects which we cannot even touch on here. It is no surprise to learn that he pioneered the artificial production of rainfall and snowfall.
Early in the war he had spent time studying the formation of clouds and icing. This required measurements of particle size using Rayleigh's law of light scattering. But how do you turn that into a tool to help win a war? Langmuir's answer was to use it to make far bigger and better smoke screens. The 'white' smoke which he and his colleague, V J Schaefer, produced became an artificial fog which protected troops during the invasion of Europe. For three days dur-ing the crossing of the Rhine a 65-mile front was blanketed with the fog. According to Langmuir's biographer, Rosenfeldl, its introduction into the Pacific by the US Navy ended the destruction caused by kamikaze attacks.
The story of the invention of this smoke screen began with a new filter for gas masks, which then needed a new smoke generator to produce smoke to test the filters. Later, when a smoke screen was needed, Langmuir and Schaefer were partway there. On 23 June, 1942, Army brass hats were dragged to the top of a suitable mountain before dawn to witness in the valley below a perfect demonstration of how to generate vast amounts of clean white smoke.
Another problem, how to prevent aircraft wings icing up, led to post-war experiments with cloud seeding. It was on 13 November, 1946, that Schaefer first seeded a cloud, using six pounds of dry ice. Langmuir reported that, 'The whole super-cooled cloud was converted to small ice crystals'. In his diary he simply wrote, 'It worked!'.
Experimental results improved and on a Winter's day that year they turned a cloud into snow. But the snow became a storm and the storm a blizzard, and the storm within G E over the legal implications led to the company waiving all royalty rights to the patents, and for the first time worrying about what Langmuir was up to.
By the time of his death at Falmouth, Massachusetts, on 16 August, 1957, Irving Langmuir's interests had spanned a range of science from electronic vacuum tubes to changing the weather. He was born in Brooklyn, New York, on 31 January, 1881, the third of four sons of Charles and Sadie Langmuir. His grandfather had emigrated from Glasgow to Canada and his father from Canada to New York, where Irving was educated at public schools in Brooklyn. He belonged to a big family; besides three brothers he had an abundant supply of aunts, uncles and cousins.
In 1892 a trip by his parents to Europe resulted in his father's appointment as the Paris representative of the New York Life Insurance Co. The four boys were shipped out to Europe and met at Le Havre by their father. Irving continued his schooling in Paris for the next three years. By that stage his love for the outdoor life, especially hiking, was well established, as was all the boys' love for dramatic chemistry experiments - chlorine excluded. Irving recalled that he had a workshop when he was nine and his own laboratory when he was 12.
He completed his schooling back in the USA, claiming that, 'Until I was 14 I always hated school and did poorly at it'. After that it was Columbia University and on to Gottingen.
Hiking was by no means his only athletic interest. He also climbed, hence his eagerness to drag the Army chiefs to the top of a mountain to witness the smoke screen demonstration. Probably his greatest climbing feat was to conquer the Matterhorn in 1921, when he was 40 years old.
Skiing was also a favourite activity, and it is said that he introduced the sport to Gottingen. In 1910, according to his biography, he did a lot of canoeing, camping and skating, took lessons in tennis, bowls, horse riding and dancing, went sleighing in the moonlight, swam, hunted, fished, and became a scout-master. His spare time was spent in the darkroom for he was a keen photo-grapher. He also became interested in that new gadget, the motor car.
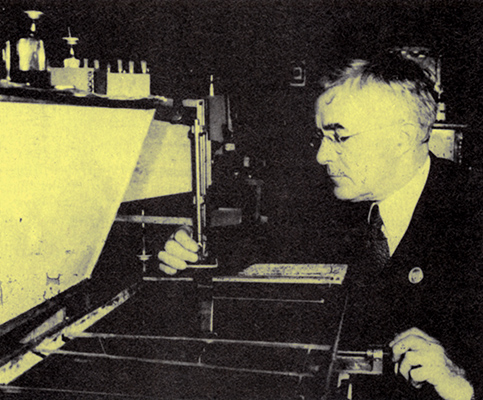
Langmuir with film balance and trough.
In his younger days, it is said that he was uncomfortable with the ladies. His diary for 29 June, 1910, records him meeting and dancing twice with a Miss Mersereau. In time he came to use her first name, Marion. They were married in 1912, forming a long and happy union in which they had two children, Kenneth and Barbara.
His wife also gave him a wonderful epitaph: 'He was as great a husband and father as he was a scientist'.
References
- A Rosenfeld, The Quintessence of Irving Langmuir, Pergamon Press, 1966.
|