|
Very few vacuum tubes have been built, as of 1948, with no glass in them. Even in the so-called 'metal tubes' the leads are brought into the tube through a glass bead sealed into an eyelet. In experimental and developmental work, glass is used even more extensively and glass-to-metal sealing assumes even greater importance.
Sealing of Small Leads into Glass
The problem of bringing leads into vacuum tubes is ever present. The principal problem involved is that of finding a metal of which the expansion coefficient matches that of the glass quite closely. Since the expansion coefficient of metals is nearly constant with temperature while that of glass generally increases with temperature, the perfect combination is seldom found. However, if the diameter of the lead is small, a considerable mismatch in expansion can be tolerated. Thus with tungsten, of which the expansion coefficient is 4 ppm (parts per million) per °C, leads of diameter 0.020 inches or less can be sealed into Pyrex glass, of which the expansion coefficient is 3.3 ppm per °C, whereas leads of diameter as great as 0.125 inches can be sealed into Nonex glass, of which the expansion coefficient is 3.6 ppm per °C, without cracking. Because the coefficient of expansion of platinum, 9 ppm per °C, is very nearly the same as that of G-12 soft lime glass, 8.7 ppm per 0C, lead size of this glass-metal combination is limited only by the budget. Platinum leads can also be sealed into G-12 soft cobalt lead glass, of which the coefficient of expansion is 8.7 ppm per °C. In all cases the glass and metal must be heated to a red heat together, bringing the glass to a soft state so that it will wet the metal. This generally requires that the metal be coated with an adherent coating of oxide and that the glass and metal be heated together so that the oxide partly dissolves in the glass, though perfect seals can be made with no oxide on copper, tungsten, or Kovar.
Metal-glass combinations other than those mentioned above may also be used for sealing small leads into glass. Dumet, which is a copper-clad iron alloy, is extensively used in receiving-tube stems of soft glass. Dumet cores are 42 per cent nickel, and the copper coating is 20 to 25 per cent of the total volume. The expansion coefficient of Dumet is close enough to that of the soft glasses so that it can be used in diameters under 0.040 inches Molybdenum can be sealed into Pyrex and Nonex in small diameters. Corning G-71, softest of the hard glasses, matches the expansion of molybdenum very closely and can be used to fairly large sizes. Some of the stainless steels have expansion coefficients low enough to be used with this same glass. Chrome-iron alloys containing 26 to 28 per cent chromium match G-6 glass quite well at low temperatures.
Copper-to-Glass Seals
Copper may be joined to almost any type of glass if the edge of the metal that is being joined to the glass is made extremely thin. This is possible in spite of the fact that the coefficient of expansion of copper is much greater than that of any of the glasses. A thin piece of copper will give to high stresses because of its high ductility and its low yield point. The technique of joining copper to glass, by using a feather edge, was perfected by Housekeeper in 1923, and such seals are often referred to as Housekeeper seals. The earliest copper to glass seals were made by Kruh and Kraus.
Copper-to-glass seals are invariably used in transmitting tubes for any seals requiring conductors larger than 0.375 inches in diameter.

Transmitting tube BW1124.
Copper is prepared for sealing by cutting or rolling the edge of copper tubing so that the edge is 1.5 ± 0.5 thousandths of an inch thick and tapered back at about a 2.5-deg angle to about 40 thousandths thickness. The joining of glass to the copper requires a high degree of skill and is probably the most difficult of all the glass working operations to perform. Small seals, up to 0.5 inches in diameter, are commonly made with the glass applied only to the inside of the copper edge. This is done because the expansion of copper is greater than that of glass and the differential expansion is therefore in the right direction to maintain the bond. For seals larger than 0.5 inches in diameter it is common to coat both the inside and the outside of the copper edge with glass. This is done primarily to prevent over oxidation of the thin copper at the seal. Copper must be heated with an oxidizing flame. The black oxide is formed, and seal temperature must be maintained constant throughout the operation. The glass is bound to the black oxide. On further heating the excess oxygen of the black oxide combines with more copper, changing it to the red oxide. Simultaneously some of the black oxide dissolves in the glass.
As a result, the final interface between the red oxide and the copper lies at a new depth, created after the glass was fused to the outside. Mechanically a rim of glass is first attached to the outside of the copper edge from a piece of glass tubing and then detached from the glass tubing, leaving a little glass projecting over the edge of the copper, which is then folded over to cover the inner side. The glass tubing is then joined to this 'bead' of glass on the copper edge. The colour of a properly fashioned copper-glass seal is a bright red and will stand heating up to the softening temperature of the glass. Seals are commonly made up to 6 inches in diameter, and some as large as 10 inches in diameter have been made. The same procedure is used in joining copper to all types of glass. Joining of copper to Pyrex is the most difficult, for there is a temperature interval of only a couple of hundred degrees between the temperature at which the glass softens and that at which the copper melts. The only disadvantages of copper-glass seals are that they are relatively difficult to make and that they have a relatively low mechanical strength because of the thinness of the copper next to the glass.
In addition to the Housekeeper seal it is possible to make disk seals to copper. This was anticipated by Housekeeper but only recently put into extensive commercial use. Disk seals are used in tubes of the lighthouse type and in reflex-klystron-oscillator tubes designed to work with external cavities. The general method of construction consists in stacking a circular copper disk with a circular hole in its centre between two equal-diameter pieces of glass and then heating the metal by torch or preferably by RF eddy currents until the copper disk becomes hot enough to melt the glass, which forms a bond with the metal.

Klystron Tube.
The glass does not cover the edges of the copper. If the copper disk is thin enough, 15 thousandths of an inch or less, then no intermediate materials are needed between the glass and copper. As is the case with the Housekeeper feather edge seal, the difference between the expansions of the glass and copper is taken up by the copper. Copper disks are frequently given a circular crimp to weaken them to radial forces and allow radial contraction without having to stretch the whole metal area. The surfaces of thick disks are often coated with a layer of copper borate, which ensures maximum bonding strength.
Kovar and Fernico
Kovar and Fernico are trade names used by the Westinghouse and General Electric Company, respectively, for some nickel-cobalt alloys of iron having non-uniform expansion characteristics that match very closely the expansion of some commercial glasses. Pure metals have expansion coefficients that are virtually independent of temperature. Ferromagnetic alloys, however, experience an increase in their expansion characteristics at the temperature at which the alloy becomes hot enough to lose its magnetic properties. The action is perfectly reversible, ie, the magnetism is restored and the coefficient of expansion reduced as the alloy is cooled. The composition of Kovar and Fernico is as follows:
Alloy
|
Iron
per cent |
Nickel
per cent |
Cobalt
per cent |
Matching
glass |
Kovar A |
53.8 |
29 |
17 |
705A0 705FN |
Fernico |
54 |
28 |
18 |
705A0 705FN |
Fernichrome |
30 |
25 |
8 |
G8 |
The difference in contraction of the principal sealing glasses and metals when cooled at a slow rate is shown below.
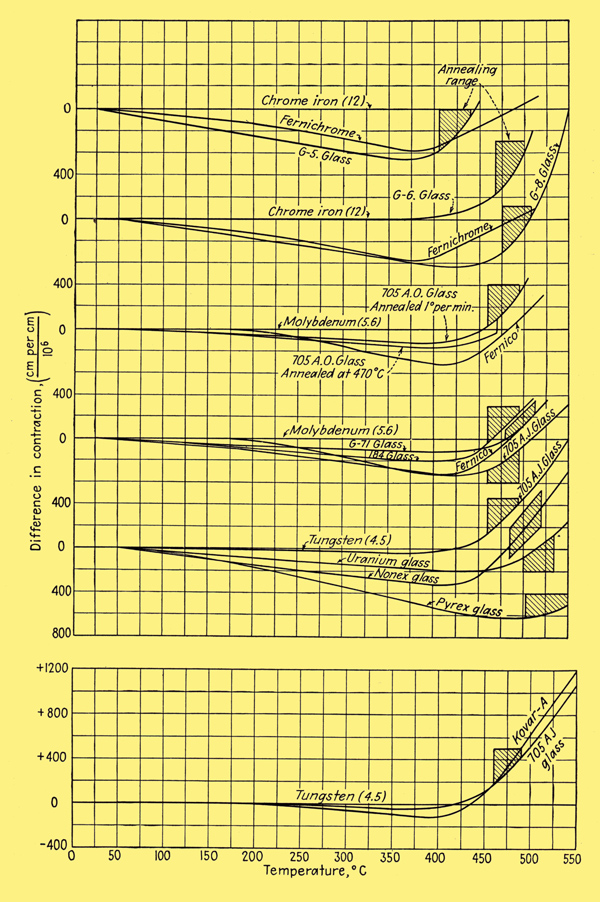
It is seen that the iron alloys match the glass characteristics quite closely over the entire temperature range. As a result, the sealing of these metals to their corresponding glasses is a relatively simple matter. No feather edges are needed; in fact, edges as thick as 1.125 inches can be joined directly. Seals as large as 4 inches in diameter can be made. Leads of Fernico wire in matching glass set in a Fernico eyelet that is welded to a metal base are used in the mass production of metal receiving tubes. As may also be seen from the image above, the reason why Nonex seals fairly successfully to tungsten is that the differential expansion is nearly zero in the annealing range. Uranium Nonex gives a better match and is sometimes used as an intermediary between tungsten and Pyrex glass.
Glass-to-Porcelain Seals
The expansion characteristics of Nonex glass and some porcelains are close enough so that Nonex can be sealed directly to porcelain. Where a porcelain-Pyrex joint is desired, Nonex should be used as an intermediary material.
|